Index
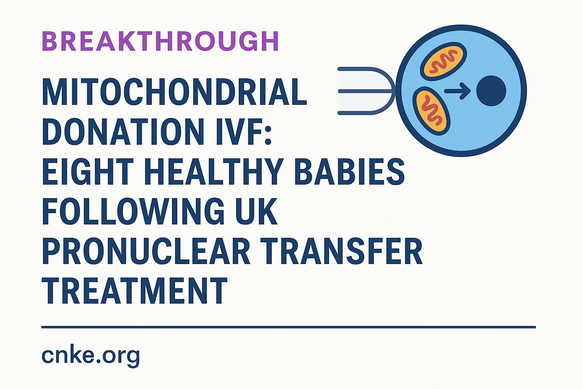
Breakthrough in Mitochondrial Donation IVF: Eight Healthy Babies Born Following UK Pronuclear Transfer Treatment
Newcastle, UK – A pioneering IVF technique designed to prevent the transmission of mitochondrial DNA disease has resulted in the birth of eight healthy babies, according to landmark studies published in The New England Journal of Medicine.
The treatment, known as pronuclear transfer (PNT), was developed by a team at Newcastle University and Newcastle upon Tyne Hospitals NHS Foundation Trust. It is the first licensed clinical application of mitochondrial donation, and was offered through the NHS Mitochondrial Reproductive Care Pathway. The outcomes represent a significant advance in reproductive medicine for families affected by mitochondrial disorders.
Clinical Context
Mitochondrial DNA (mtDNA) diseases affect approximately 1 in 5,000 live births. These maternally inherited disorders impair energy production, particularly in organs with high energy demands such as the brain, heart, and muscle. With no curative treatment currently available, IVF-based risk-reduction technologies such as PNT offer an innovative reproductive option.
Methodology: Pronuclear Transfer
PNT involves removing the nuclear genome from a fertilised egg carrying mutated mtDNA and transferring it into a donor fertilised egg with healthy mitochondria and removed nuclear DNA. This results in embryos with nuclear DNA from the intended parents and mitochondrial DNA from the donor, effectively reducing the risk of disease transmission.
Clinical Outcomes
- 22 patients underwent PNT; 8 clinical pregnancies confirmed; 8 live births reported.
- All children were born healthy, including one set of identical twins.
- Five children had undetectable levels of pathogenic mtDNA mutations.
- Three children had low-level mtDNA mutations (5–20%)—well below the ~80% threshold associated with clinical disease.
- All babies are meeting developmental milestones and continue to be followed as part of an 18-month developmental study.
The authors highlight that minor neonatal health issues observed (e.g., transient startles, hyperlipidaemia, arrhythmia, UTI) were resolved with standard care and are unlikely to be related to the mitochondrial donation procedure or mtDNA mutation load.
Reproductive Care and Monitoring
All mothers underwent close monitoring during pregnancy. Six of seven progressed without incident; one developed hyperlipidaemia, managed successfully with dietary modification. Deliveries were via vaginal or elective caesarean section. All neonates were born at appropriate weights for gestational age.
Integrated Risk-Reduction Strategy
The Newcastle team offered PNT only when preimplantation genetic testing (PGT) alone was insufficient. Comparatively, PGT alone resulted in 18 live births from 39 patients. PNT is thus integrated into a comprehensive, regulated reproductive care pathway under HFEA licensing.
Expert Commentary
Professor Mary Herbert, lead author: “The findings give grounds for optimism. However, understanding and mitigating the carryover of maternal mitochondria is critical to improving outcomes.”
Professor Bobby McFarland, first author: “While long-term follow-up is essential, these early outcomes are very encouraging. It’s a privilege to witness the impact this has on families.”
Professor Sir Doug Turnbull: “Mitochondrial disease can devastate families. This breakthrough offers new hope to women at risk of passing on these conditions.”
Future Directions
Follow-up to age five is underway to monitor child development and detect any late-emerging effects. Continued research aims to refine the PNT technique, reduce mitochondrial carryover, and close the gap between risk reduction and disease prevention.
Resources for Clinicians and Researchers
- Mitochondrial Donation and PGT – NEJM Article
- Mitochondrial Donation in a Reproductive Care Pathway – NEJM Article
- HFEA: Mitochondrial Donation Treatment
- NHS Mitochondrial Services
- The Lily Foundation – UK Mitochondrial Disease Charity
About the Programme
- Legalisation: UK legalised mitochondrial donation in 2015 after scientific and ethical review; Australia has since followed.
- Licensing: Newcastle Fertility Centre was first globally licensed to perform PNT in 2017.
- Support: Programme funded by NHS England, Wellcome, and NIHR, with infrastructure support from Newcastle University.
“We now know that eight babies have been born using this technique, all showing no signs of mito. For many affected families, it’s the first real hope of breaking the cycle of this inherited condition.”
— Liz Curtis, CEO, The Lily Foundation