Researchers from the University of Bonn and DZNE uncover the role of brain inflammation in spastic paraplegia type 15, offering new therapeutic possibilities.
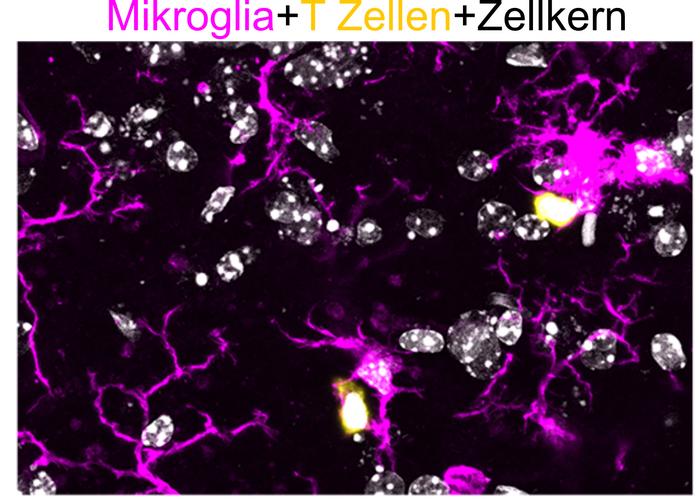 Microglia (Magenta) interacting with T cells (yellow) in the central nervous system of SPG15-deficient mice. Cell nuclei are shown in grey. A groundbreaking study led by the University of Bonn and the German Center for Neurodegenerative Diseases (DZNE) has revealed that early-stage inflammation in the brain, driven by immune cells, plays a key role in the development of spastic paraplegia type 15 (SPG15), a rare hereditary movement disorder. The research, recently published in the Journal of Experimental Medicine, underscores the intricate connection between the immune system and neurodegeneration, highlighting avenues for new therapeutic strategies.
Microglia (Magenta) interacting with T cells (yellow) in the central nervous system of SPG15-deficient mice. Cell nuclei are shown in grey. A groundbreaking study led by the University of Bonn and the German Center for Neurodegenerative Diseases (DZNE) has revealed that early-stage inflammation in the brain, driven by immune cells, plays a key role in the development of spastic paraplegia type 15 (SPG15), a rare hereditary movement disorder. The research, recently published in the Journal of Experimental Medicine, underscores the intricate connection between the immune system and neurodegeneration, highlighting avenues for new therapeutic strategies.
Understanding SPG15: A Devastating Childhood-Onset Disorder
Spastic paraplegia type 15 is marked by progressive degeneration of motor neurons, with symptoms such as leg weakness, uncontrollable twitching, and eventual paralysis often emerging during adolescence. Until now, the mechanisms underlying the loss of these critical neurons remained poorly understood.
The study, spearheaded by Professor Elvira Mass (University of Bonn), Dr. Marc Beyer (DZNE), and Professor Ralf Stumm (University Hospital Jena), focused on the immune system's contribution to disease onset. Using a murine model genetically engineered to mimic SPG15, the team uncovered profound changes in brain-resident immune cells—microglia—long before any overt neuronal damage occurred.
A Hidden Battle Within: Microglia and T Cells
Employing advanced single-cell imaging techniques, the researchers distinguished between microglia and bone marrow-derived immune cells using fluorescent markers. They observed that microglia in the early stages of SPG15 transform into "disease-associated microglia," releasing signals that attract cytotoxic CD8+ T cells from the bloodstream. This immune interaction amplifies inflammation, setting the stage for subsequent neuronal death.
“Our data suggest that the early stages of the disease are driven not by the initial loss of motor neurons but by an aggressive immune response,” said Professor Mass. “This finding offers a paradigm shift in how we understand and potentially treat SPG15.”
Implications for Broader Neurodegenerative Research
While SPG15 is distinct from conditions like Alzheimer’s disease, the parallels in immune dysregulation are striking. The researchers note that similar early inflammatory cascades may underpin more common neurodegenerative disorders, thus broadening the significance of their findings.
“By bridging immunology and neurobiology with state-of-the-art single-cell technology, we were able to illuminate an underexplored aspect of disease pathogenesis,” Dr. Beyer explained. “This interdisciplinary approach paves the way for future studies and interventions.”
Hope for Future Therapies
The study raises the promising prospect of using immune-modulating therapies to slow disease progression. Immune suppression at early stages could offer tangible benefits to patients, potentially delaying the onset of debilitating symptoms.
This collaborative effort, part of the ImmunoSensation2 Cluster of Excellence, involved institutions from Bonn, Jena, and Melbourne, with funding support from the German Research Foundation (DFG), the Federal Ministry of Education and Research (BMBF), and the European Research Council (ERC).
Reference
Frolov A, et al. Microglia and CD8+ T cell activation precede neuronal loss in a murine model of spastic paraplegia 15. Journal of Experimental Medicine. DOI: https://doi.org/10.1084/jem.20232357
Cover Image: Bordellszene (1916); Ernst Ludwig Kirchner (German, 1880-1938)